Have you ever stared at your pool test results feeling completely overwhelmed, wondering if you need a chemistry degree just to keep your water safe and sparkling? You’re not alone – thousands of pool owners struggle daily with the mysterious world of pool chemical balance, turning what should be a relaxing backyard oasis into a source of constant stress and frustration.
The truth is, mastering pool chemistry isn’t rocket science, but it does require understanding the delicate dance between different chemical components that keep your water crystal clear and swimmer-safe. When your pool chemical balance is off, you’re not just dealing with cloudy water – you’re potentially exposing your family to harmful bacteria, damaging expensive equipment, and throwing money down the drain on ineffective treatments.
This comprehensive guide will transform you from a confused pool owner into a confident water chemistry expert. You’ll discover the exact formulas professionals use, learn to read your water like a book, and gain the insider knowledge that will save you hundreds of dollars in unnecessary chemicals while ensuring your pool remains a safe, inviting sanctuary all season long.
Pool Chemical Balance
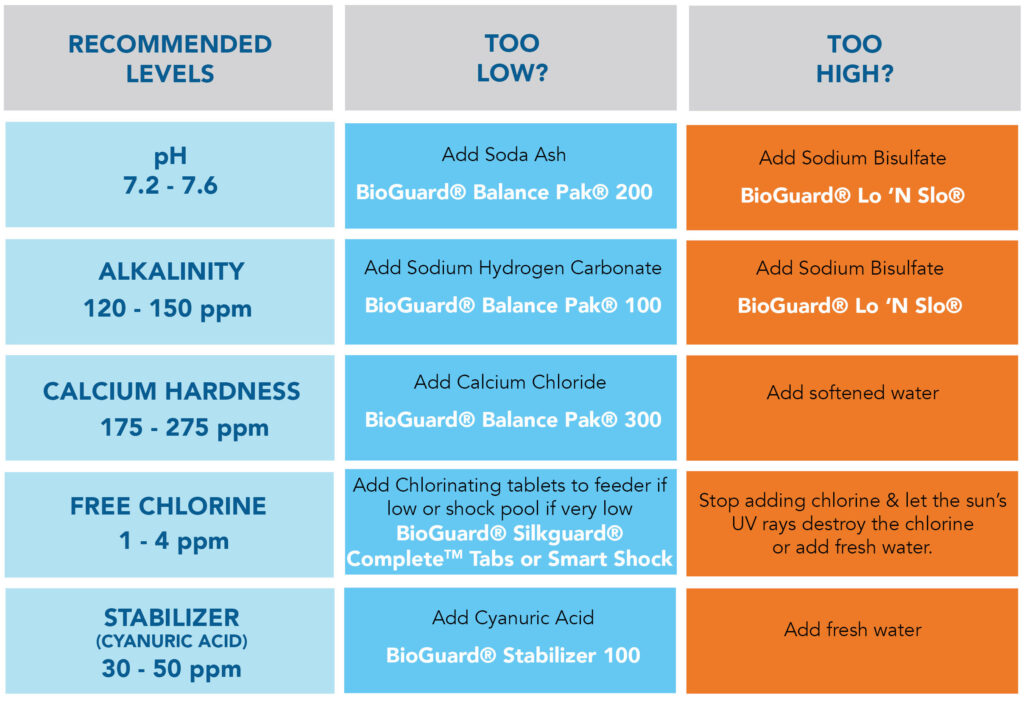
- Pool chemical balance requires maintaining four critical parameters: pH levels, chlorine residual, total alkalinity, and calcium hardness within specific ranges
- pH levels between 7.2-7.6 optimize chlorine effectiveness and prevent equipment damage or swimmer discomfort
- Free chlorine should maintain 1.0-3.0 ppm for residential pools, with higher levels needed during heavy use or algae treatment
- Total alkalinity acts as a pH buffer and should be maintained between 80-120 ppm for optimal water stability
- Calcium hardness prevents corrosive water conditions and should range from 150-300 ppm depending on pool surface type
- Testing frequency should increase during peak season, with weekly testing as the minimum standard for safe swimming
- Understanding chemical interactions prevents common mistakes that waste money and compromise water quality
The Foundation of Perfect Pool Water
Pool chemical balance represents far more than simply adding chlorine and hoping for the best. Think of your pool as a complex ecosystem where every chemical component influences the others, creating either harmony or chaos depending on your understanding and management skills.
The concept of balance means maintaining all chemical parameters within their ideal ranges simultaneously. When one parameter drifts outside its optimal zone, it creates a domino effect that impacts every other aspect of your water chemistry. This interconnected relationship explains why experienced pool professionals test multiple parameters rather than focusing on just chlorine levels.
Professional pool technicians understand that water chemistry follows predictable patterns and scientific principles. By mastering these fundamentals, you can anticipate problems before they develop, maintain consistently clear water, and dramatically reduce your chemical costs through efficient, targeted treatments.
The Big Four: Essential Chemical Parameters
Every successful pool chemical balance program focuses on four critical measurements that determine your water’s safety, clarity, and equipment compatibility. These parameters work together like instruments in an orchestra – when one is out of tune, the entire performance suffers.
pH levels serve as the conductor of your pool’s chemical orchestra, influencing every other parameter’s effectiveness. When pH climbs too high, chlorine becomes lazy and ineffective, allowing bacteria and algae to flourish despite adequate sanitizer levels. When pH drops too low, chlorine becomes overly aggressive, causing equipment corrosion and swimmer irritation.
Free available chlorine provides the sanitizing power that keeps your water safe for swimming. This measurement represents the active chlorine available to kill bacteria, viruses, and other contaminants. Understanding the difference between free chlorine and total chlorine helps you make informed decisions about when and how much sanitizer to add.
Mastering pH: The Key to Everything Else

pH levels control the effectiveness of every other chemical in your pool, making this single parameter the most critical aspect of pool chemical balance. The pH scale ranges from 0 to 14, with 7.0 representing neutral water. Pool water should maintain a slightly basic pH between 7.2 and 7.6 for optimal performance.
When pH rises above 7.6, several problems emerge simultaneously. Chlorine efficiency drops dramatically, requiring two to three times more sanitizer to achieve the same disinfection level. Calcium begins precipitating from solution, creating scale deposits on pool surfaces and equipment. Swimmers experience burning eyes and skin irritation despite proper chlorine levels.
Conversely, when pH falls below 7.2, your pool water becomes aggressive and corrosive. Metal components begin dissolving, potentially staining pool surfaces and damaging expensive equipment. Chlorine becomes overly active, causing rapid dissipation and requiring frequent additions to maintain proper sanitizer levels.
The Science Behind pH Control
Understanding the buffer system that controls pH levels empowers you to maintain stable water chemistry with minimal chemical additions. Total alkalinity acts as your pool’s pH buffer, resisting sudden changes that could disrupt your pool chemical balance.
Think of total alkalinity as a shock absorber for pH fluctuations. When alkalinity levels fall below 80 ppm, pH becomes unstable and difficult to control, bouncing up and down with minor chemical additions or environmental changes. When alkalinity exceeds 120 ppm, pH becomes locked in place, resisting adjustment even with large chemical additions.
The relationship between pH and alkalinity creates a balancing act that requires understanding their interaction. Raising alkalinity typically increases pH, while lowering alkalinity usually decreases pH. Professional pool technicians leverage this relationship to make efficient adjustments that address both parameters simultaneously.
| pH Range | Chlorine Effectiveness | Water Condition | Action Required |
|---|---|---|---|
| 7.0-7.2 | 80% effective | Slightly aggressive | Monitor closely |
| 7.2-7.6 | 100% effective | Ideal balance | Maintain current levels |
| 7.6-8.0 | 40% effective | Scale-forming | Lower pH immediately |
Chlorine Mastery: Beyond Basic Sanitization
Free chlorine represents the active sanitizing power in your pool, but understanding chlorine chemistry requires knowledge that goes far beyond simply adding liquid or granular sanitizer. The chlorine family includes several different compounds, each with unique characteristics that affect your pool chemical balance differently.
Sodium hypochlorite (liquid chlorine) provides pure sanitizing power without adding other chemicals to your water. This makes it ideal for pools with high total dissolved solids or those requiring precise chemical control. However, liquid chlorine has a high pH (around 13), requiring pH adjustment after each addition.
Calcium hypochlorite (granular shock) delivers powerful sanitizing action while adding calcium to your water. This dual action benefits pools with low calcium hardness but can create problems in areas with naturally hard water. The high available chlorine content (65-70%) makes it economical for large pools or heavy sanitizer demand.
“The biggest mistake pool owners make is thinking all chlorine products are the same. Each type affects your water balance differently, and choosing the wrong one can create expensive problems down the road.” – Certified Pool Operator
Advanced Chlorine Management Techniques
Professional pool chemical balance management involves understanding chlorine demand, breakpoint chlorination, and combined chlorine management. These advanced concepts separate successful pool owners from those who struggle with persistent water problems.
Chlorine demand refers to the amount of sanitizer required to achieve a measurable free chlorine residual. New pools or those with organic contamination may have high chlorine demand, consuming large amounts of sanitizer before establishing a proper residual. Understanding this concept prevents panic when initial chlorine additions seem to disappear immediately.
Breakpoint chlorination represents the process of adding enough chlorine to eliminate combined chlorines (chloramines) that cause strong chemical odors and reduce sanitizing effectiveness. This process typically requires adding 10 times the combined chlorine reading in additional free chlorine, temporarily creating very high sanitizer levels.
READ ALSO: Pool Shock Treatment Explained: When, Why, and How to Shock Your Pool for Crystal Clear Water
Total Alkalinity: Your Pool’s Stability Buffer
Total alkalinity serves as the unsung hero of pool chemical balance, providing the stability that keeps pH levels manageable and predictable. This parameter measures your water’s ability to resist pH changes, acting as a chemical buffer that smooths out the natural fluctuations that occur in pool water.
Low alkalinity creates pH instability that makes water chemistry management nearly impossible. Small chemical additions cause dramatic pH swings, equipment damage, and swimmer discomfort. Pool owners with low alkalinity often find themselves constantly adjusting pH without achieving lasting stability.
High alkalinity creates the opposite problem – pH becomes locked in place and resistant to adjustment. This condition, known as pH lock, prevents effective chemical management and can trap pH at levels that reduce chlorine effectiveness or cause equipment damage.
Alkalinity Adjustment Strategies

Raising alkalinity requires sodium bicarbonate (baking soda) additions, while lowering alkalinity involves careful acid additions combined with aeration to drive off excess carbonates. The key to successful alkalinity management lies in making gradual adjustments over several days rather than attempting dramatic corrections.
When raising alkalinity, add sodium bicarbonate gradually over 2-3 days, allowing each addition to circulate completely before retesting. This approach prevents overcorrection and allows you to fine-tune the adjustment as you approach your target range.
Lowering alkalinity requires a more complex process involving muriatic acid additions followed by vigorous aeration. Add acid to reduce both alkalinity and pH, then use aeration (running fountains, spa jets, or air compressors) to raise pH back to normal while leaving alkalinity at the reduced level.
Calcium Hardness: Protecting Your Investment
Calcium hardness represents one of the most misunderstood aspects of pool chemical balance, yet it plays a crucial role in protecting your pool surfaces and equipment from damage. This parameter measures the amount of dissolved calcium in your water, with proper levels preventing both corrosive and scale-forming conditions.
Low calcium hardness creates aggressively corrosive water that dissolves calcium from pool surfaces, grout, and equipment. This condition, known as hungry water, literally eats away at your pool infrastructure, causing expensive damage that may not become apparent for months or years.
High calcium hardness leads to scale formation as excess calcium precipitates from solution and deposits on surfaces and equipment. These scale deposits reduce equipment efficiency, create rough surfaces that harbor bacteria, and can permanently damage pool finishes.
Balancing Act: The Langelier Saturation Index
Professional pool technicians use the Langelier Saturation Index (LSI) to evaluate the interaction between pH, alkalinity, calcium hardness, and temperature. This calculation determines whether your water is balanced, aggressive, or scale-forming under current conditions.
The LSI calculation considers all four parameters plus water temperature to predict your water’s behavior. Negative LSI values indicate aggressive water that will dissolve calcium, while positive values suggest scale-forming tendencies. The goal is achieving an LSI between -0.3 and +0.3 for optimal water balance.
Understanding LSI empowers you to make informed decisions about chemical adjustments based on your specific water conditions and climate. This knowledge prevents costly mistakes and ensures your pool chemical balance program protects both water quality and equipment longevity.
Testing and Monitoring: Your Chemical Compass

Accurate testing forms the foundation of successful pool chemical balance management. Without reliable measurements, you’re essentially flying blind, making chemical adjustments based on guesswork rather than scientific data.
Digital test meters provide superior accuracy compared to test strips, especially for critical parameters like pH and chlorine. While test strips offer convenience for quick checks, their accuracy limitations make them unsuitable for precise chemical management in challenging conditions.
Professional-grade test kits using liquid reagents deliver laboratory-quality results that enable confident chemical decisions. These kits cost more initially but provide superior accuracy and reliability that saves money through more efficient chemical usage.
Testing Frequency and Timing
Pool chemical balance requires different testing frequencies based on usage patterns, weather conditions, and seasonal factors. During peak swimming season, test at least twice weekly, with daily testing during periods of heavy use or adverse weather.
Test timing affects result accuracy and usefulness. Test in the evening after daily swimming activities conclude but before adding any chemicals. This timing provides the most accurate picture of your water’s current condition and chemical demand.
- Morning testing shows overnight chemical loss and helps plan daily adjustments
- Pre-swim testing ensures safe conditions before pool use begins
- Post-swim testing reveals the impact of bather load on water chemistry
- Evening testing provides baseline readings for chemical planning
- Storm testing identifies weather-related chemical changes requiring attention
Professional Troubleshooting Techniques
Even experienced pool owners occasionally encounter pool chemical balance challenges that require advanced troubleshooting skills. Understanding common problems and their underlying causes enables quick resolution and prevents minor issues from becoming major headaches.
Persistent cloudiness despite proper chemical levels often indicates filtration problems or the presence of dissolved solids that interfere with normal chemical processes. This condition requires systematic evaluation of filtration efficiency, chemical interactions, and potential contamination sources.
Chemical lock occurs when one parameter prevents others from responding to adjustment attempts. This frustrating condition typically involves alkalinity levels that resist pH changes or combined chlorine levels that prevent free chlorine establishment. Breaking chemical lock requires specific strategies that address the underlying parameter relationships.
Advanced Chemical Interactions
Understanding how different chemicals interact prevents common mistakes that waste money and create water problems. Adding multiple chemicals simultaneously can cause precipitation reactions that render treatments ineffective while creating new problems.
Calcium and bicarbonate interactions become critical when adjusting both alkalinity and hardness simultaneously. Adding these chemicals together can cause cloudiness or precipitation that requires additional treatment to resolve.
Metal sequestrants and chlorine interact in ways that can either enhance or reduce sanitizer effectiveness. Timing these additions properly maximizes their individual benefits while preventing negative interactions that compromise water quality.
Your Path to Pool Chemistry Mastery
Mastering pool chemical balance transforms pool ownership from a source of stress into a rewarding hobby that provides years of safe, enjoyable swimming. The key lies in understanding the scientific principles that govern water chemistry rather than relying on trial-and-error approaches that waste time and money.
Remember that achieving perfect pool chemical balance is a journey, not a destination. Water chemistry constantly evolves based on usage, weather, and environmental factors, requiring ongoing attention and adjustment. However, the knowledge you’ve gained here provides the foundation for confident decision-making in any situation.
Start implementing these professional techniques gradually, focusing on one parameter at a time until you’ve mastered the entire system. Keep detailed records of your test results and chemical additions to identify patterns that will help you anticipate and prevent problems before they develop. With practice and patience, you’ll soon be managing your pool chemistry like a seasoned professional.
People also ask
1. In what order should I balance my pool chemicals?
To effectively balance pool chemicals, it’s generally recommended to start by adjusting the total alkalinity (TA). Then, you should adjust the pH, followed by calcium hardness, and finally add your chosen disinfectant (chlorine or bromine). Adding algaecide and shocking the pool are also important steps.
2. What happens if you swim in a chemically unbalanced pool?
Chemical imbalance can cause irritations and infections
The most significant risks associated with unbalanced chemicals are skin and eye irritation and infections. High chlorine levels, for example, can cause redness and burning of the eyes and skin, while low levels can allow bacteria to grow, leading to infections.
3. What does it mean when pool chemicals are balanced but water is still cloudy?
If the chemicals are balanced but you still have cloudy pool water then it’s likely caused by particles. With everything else as it should be, a water clarifier can easily help gather the particles for collection by the pool’s filter. Another way to clear the particles out of your pool water is using pool flocculant.
4. How long does it take to chemically balance a pool?
It typically takes up to 24 hours to properly balance pool chemicals. However, the exact time can vary depending on factors like pool size, water condition, and the specific adjustments needed. For example, if you’re raising alkalinity using baking soda, you should wait at least six hours before retesting, according to Latham Pool.
5. Does rain dilute pool chemicals?
Rainwater can have a significant impact on your pool’s chemical balance. It dilutes the chemicals in your pool, reducing their effectiveness in sanitizing the water and eliminating harmful bacteria. Additionally, rain can introduce contaminants like dirt, debris, and even algae spores, further complicating the balance.

